Vaccines are a powerful public health tool and the bedrock of sustainable epidemic control.
Vaccines are a powerful public health tool and the bedrock of sustainable epidemic control.
The Role of Vaccines in Prevention
HIV
A safe, effective and accessible HIV vaccine is urgently needed to provide a durable end to the pandemic. Designing a vaccine against HIV is exceptionally challenging, given the complicated and ever-changing nature of the virus. Still, much progress has been made, with research providing more and more clues to an effective approach. Ending AIDS requires simultaneous action on multiple fronts of research, development, and delivery. AVAC continues to advocate for HIV vaccine R&D, expanded access to existing prevention options, and development of new user-centered prevention strategies.
Other STIs
The development of vaccines, and other biomedical interventions such as diagnostics and pre- and post-exposure prophylaxis, could invigorate efforts to address the global problem of sexually transmitted infections (STIs). Vaccines currently exist for two common STIs – HPV and hepatitis B. While there are cures for some STIs (syphilis, gonorrhea, chlamydia and trichomoniasis), many STI infections go undiagnosed and untreated, either because they cause few or no symptoms or because diagnostics remain difficult to access.
COVID-19
Vaccines have played an important role in reducing the severity of COVID-19 infections. Research on vaccines that reduce disease or block transmission is ongoing, as is the development of novel technology for vaccines against COVID-19.
The Research Pipeline
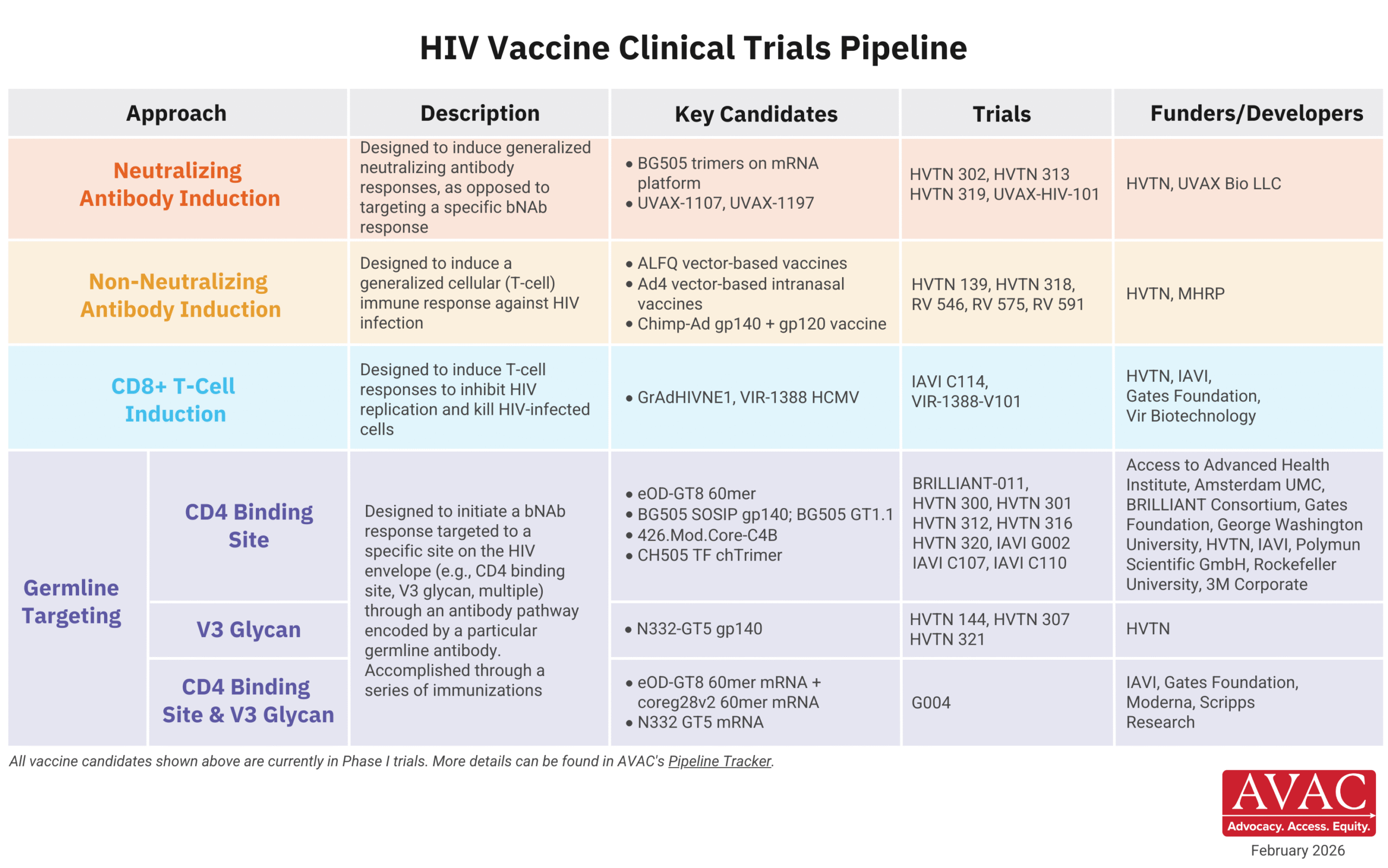
HIV vaccine R&D is actively focused on early-stage research, with the portfolio centering primarily on small, iterative discovery medicine trials to develop candidates to induce broadly neutralizing antibodies (bNAbs), as well as Phase I trials testing T-cell-inducing candidates. Lessons from large scale trials that did not show efficacy have informed this shift in strategy from traditional product development to discovery medicine.
Investment in HIV vaccine R&D has transformed immune science and provided the foundation for major advances across disease areas. Continued investment in this pipeline is essential to the advance of human health.
STIs
Vaccines currently exist for only two common STIs – HPV and hepatitis B — with research underway for vaccines against gonorrhea, genital herpes, chlamydia, and others. Go to STIwatch.org to learn more.
COVID-19
Research and development is ongoing to develop next generation vaccines against coronaviruses and its COVID-19 strains.
Vaccine Access: HIV, STIs, COVID & Beyond
The COVID pandemic made stark long-standing disparities in access to life-saving interventions, when rich countries and communities benefited from vaccines while low- and middle-income countries and communities struggled through much of the pandemic without them. HIV advocates have called out such disparities for decades. Questions of access must rise to the top of the agenda for advocates, policymakers, and funders alike. Sustained, robust advocacy for access is core to equity in global health.
In addition, well-resourced, evidence-based proactive strategies must be in place to out-maneuver misinformation and disinformation, and to redress vaccine hesitancy. This advocacy is vital for vaccines, against HIV, COVID, STIs, and other health threats.
Vaccine Advocacy
Political and public support for the role of vaccines in public health has eroded under ideological attacks from vaccine deniers. Funding cuts to vaccine R&D, across disease areas and for HIV specifically, represent a grave threat to advancing vaccine science.
It’s imperative to broaden and deepen public understanding of the value of vaccines in public health and demand political leaders fight for sustained and robust funding in vaccine research. Community-led education, communication, and advocacy to counter misinformation and disinformation is crucial to building trust and addressing vaccine hesitancy in diverse communities.
HIV
Vaccine strategies currently being explored have a long way to go and support for these efforts is essential. Advocacy for Good Participatory Practice in research and development is as important as ever.
STIs
A history of underfunding this crucial area of public health must end. Advocates, researchers, affected communities, policymakers, and funders should be making connections, embracing Good Participatory Practice and prioritizing STI vaccine development and implementation.
COVID-19
With next generation coronavirus vaccine development underway, public funding for these efforts can speed innovation. Such investment must be matched with political commitments to equitably deliver products that prove successful.
Vaccine Research and Global Health Equity
Vaccine development has cascading benefits across public health. Advances against one health threat inform research against others. But tremendous scientific progress against viruses such as HIV and COVID-19 must be matched with global commitments to deliver proven vaccines, and other critical health tools for diagnosis, prevention and treatment, to the world’s most vulnerable populations, no matter where they live. It’s imperative to empower all countries to equitably contribute and share in the fruits of science.
People’s Research Agenda
Our People’s Research Agenda offers a state of the field update on HIV vaccine R&D, which is actively focused on early-stage research, with the portfolio centering primarily on small, iterative discovery medicine trials to develop germline-targeting candidates to induce bNAbs, as well as Phase I trials testing T-cell-inducing candidates.