October 9, 2025
This roundup from AVAC highlights key developments across the field of HIV prevention from accelerating access to injectable lenacapavir (LEN) for PrEP, to advancing inclusion of pregnant and lactating people, and trans and gender-diverse communities in research. It also features a new initiative reinforcing the highest standards in ethical research conduct and tools for the fight to sustain funding in HIV research.
Read on for the details!
The Latest on Speed, Scale and Equity in the Rollout of LEN for PrEP
In the midst of profound disruptions to US leadership in global health, the world has seen sustained momentum behind the rollout of injectable LEN for PrEP.
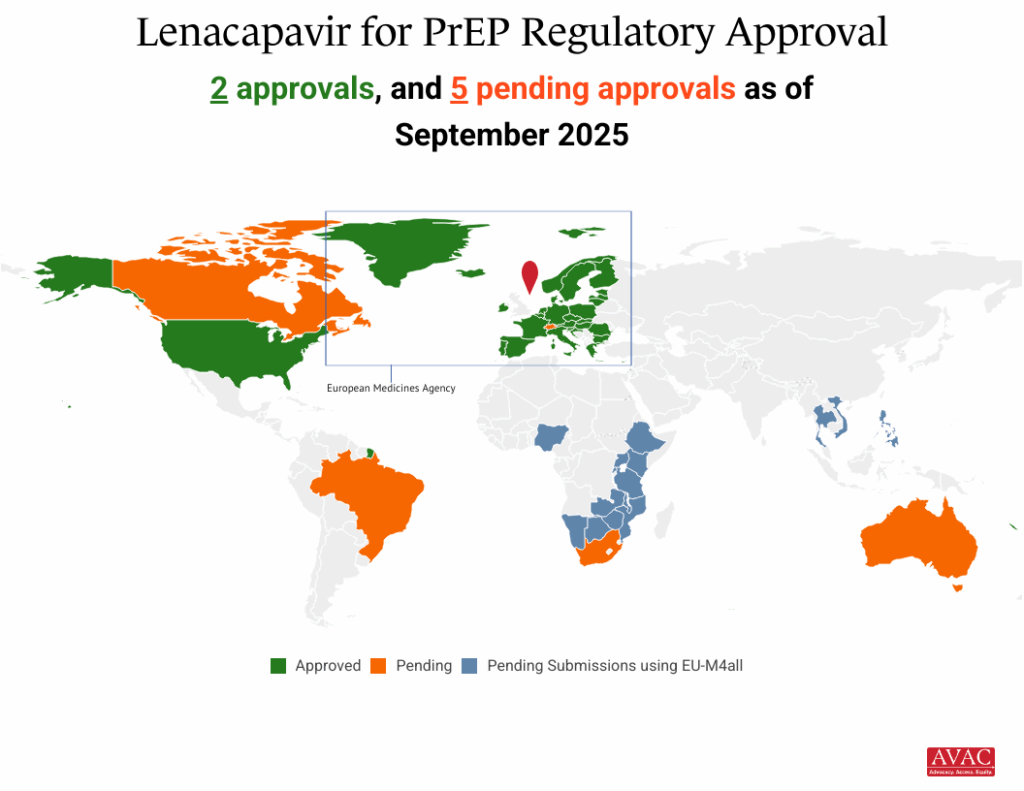
The latest news came on October 6, with the WHO issuing a prequalification for Gilead’s lenacapavir pill and injection products using a new “abridged prequalification pathway” that took just 36 days. WHO prequalification, based on the positive scientific opinion of the European Medicines Agency (EMA), adds lenacapavir to a list of products evaluated as safe, effective, and crucial for improving public health. Speeding up evaluation and regulatory approvals has been a long-standing advocacy priority in HIV prevention, and spurs momentum toward scaling up LEN for PrEP.
AVAC’s updated infographics and resources feature the latest information on the price and the process for accelerating access:
- Lenacapavir Regulatory Approval
- Potential Demand for LEN for PrEP
- HIV Prevention Product Overview
- Where We Are Now With LEN
- PrEP Price Comparison
- Factsheets on action steps for stakeholders to take now and other essential resources on AVAC’s dedicated page on advocacy for the ethical rollout of LEN for PrEP
WHO Launches Global Clinical Trials Forum
This week, the World Health Organization launched the Global Clinical Trials Forum (GCTF), a global network to strengthen clinical trial environments and infrastructure at national, regional and global levels. The GCTF has shown vision and commitment to the ethical conduct of research by incorporating the Good Participatory Practice Guidelines into its work.
“The work of the Global Clinical Trials Forum (GCTF) is exciting and necessary. Best practices in clinical trials can be strengthened and sustained with meaningful and informed guidance and coordination such as that under the GCTF. AVAC applauds the WHO’s ongoing commitment to the Good Participatory Practice Guidelines within the GCTF, which ensure ethical, inclusive and effective stakeholder engagement in research. We are proud to be founding member.” — Cindra Feuer, AVAC Senior Program Manager
Advancing the Inclusion of Pregnant and Lactating Populations in Research
Cathy Slack of HAVEG, AVAC’s Breanne Lievense, former AVACer Manju Chatani and partners published a review of ethics guidelines supporting access to HIV clinical trials for pregnant and lactating individuals, a population too often excluded from research. Find related resources, including an Advocate’s Guide on the issue created as part of the Coalition to Accelerate & Support Prevention Research (CASPR), on AVAC’s dedicated page.
A Scorecard for Including Trans and Gender Diverse Communities in HIV Prevention Research
The American Journal of Public Health just published Addressing Transgender Erasure in HIV Clinical Trials: The Scorecard for Transgender and Gender-Diverse Inclusion, by AVAC’s Cindra Feuer and Brian Minalga of HANC. In it, they share findings from this first cross-sectional review of trans participation in HIV clinical trials and chart the movement from TGD invisibility to a recent uptick of inclusion. In addition to the article, Cindra and Brian’s blog, Inclusion Made Simple, In Difficult Times, puts these findings in the context of the US policy attacks on TGD people. Find additional advocacy resources at avac.org/transgender-manifesto.
Upcoming Webinar
And coming up, don’t miss The Choice Agenda & AVAC hosted webinar on this critical issue, Do Not Check That Box—Impacts From the Assault on Transgender Communities and DEI + Strategies to Sustain and Rebuild.
Advocating for Sustained Investment in HIV Prevention Research
The field of HIV prevention is confronting an unpreceded need to defend sustained investment in research. AVAC and our partners have been meeting the moment—these resources can support advocacy across the field.
- AVAC input on the re-competition of the NIAID HIV/AIDS Clinical Trials Networks
- The People’s Research Agenda
- 24 Hours to SAVE AIDS Research
- HIV Prevention R&D at Risk: Tracking the Impact of US Funding Cuts
We hope these resources support our collective advocacy. We know the scope of the threats to HIV prevention research and global health are broad and deep. But the fight is ours to win, with an abiding passion and commitment from fierce advocates. Together we have already achieved major gains against HIV, and the fight continues.
Jirair Ratevosian said it well: “Hope isn’t naïve—it’s an act of defiance. And in a moment like this, choosing hope is its own kind of leadership.”