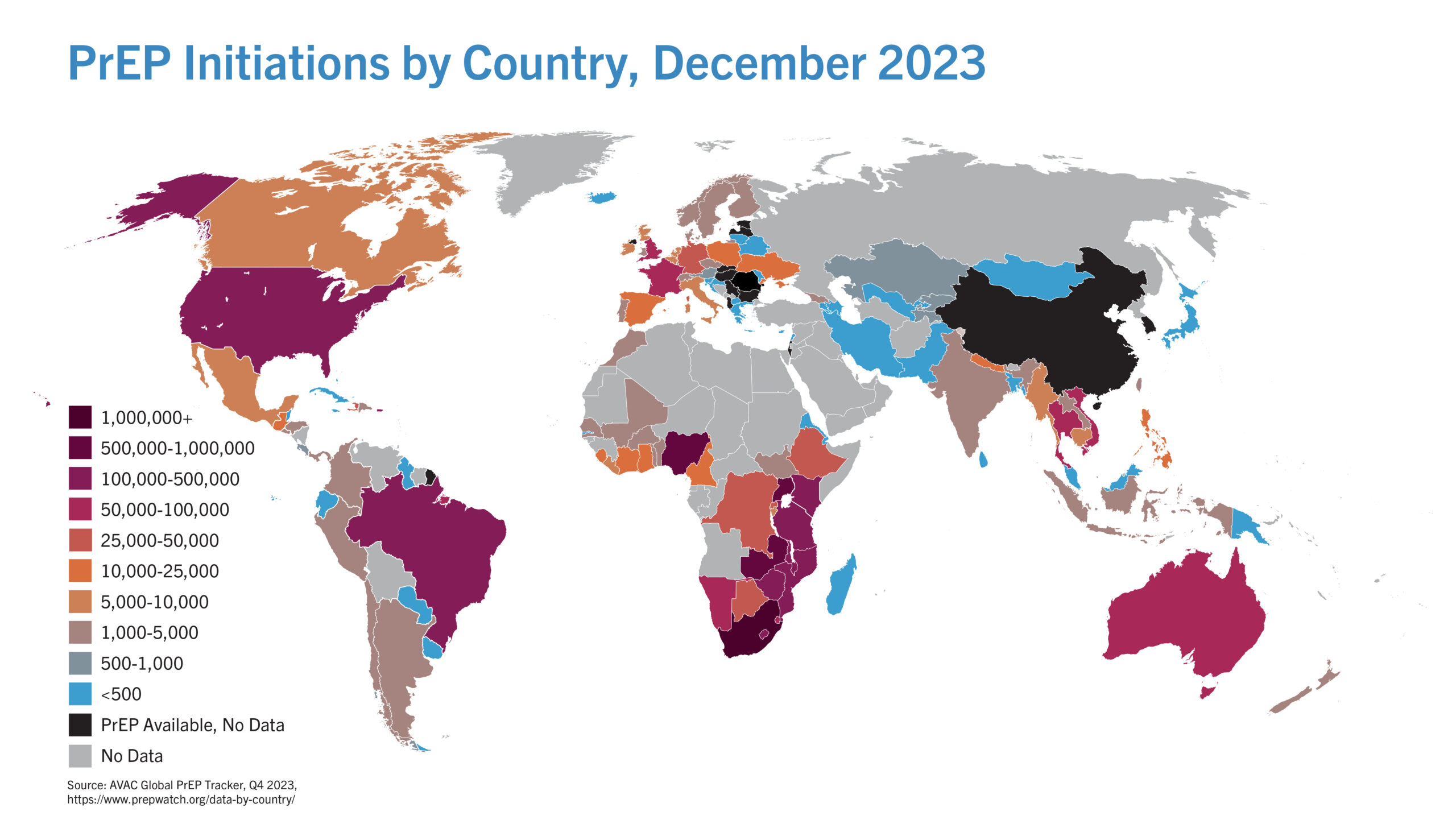
We champion the Good Participatory Guidelines and we connect global and national decision-makers to a network of research-savvy advocates who prioritize community voices and bring accountability to prevention science and policy.
More than 40 years of HIV prevention advocacy has demonstrated the essential role communities must play for the world to achieve epidemic control and advance global health. The history of HIV advocacy is unique; affected communities came together calling out their needs and demanding justice. The result has been a smarter, more equitable, more effective response to a global health threat than ever seen before.
AVAC connects the global response to the real needs of affected people, and we do this by:
- working in solidarity with communities to understand, bring attention to and support for efforts to address the root causes of inequity that inform and imperil HIV prevention and global health;
- connecting decision-makers to a global network of advocates to put the perspectives of affected communities at the center of research and policy;
- supporting communities to understand the science and process of R & D and policy making; and
- creating platforms for discussion, debate and advocacy.
Good Participatory Practice (GPP) is a flagship of this work. Guidelines AVAC developed in partnership with UNAIDS in 2007 provide a global standard for broad and inclusive stakeholder engagement in clinical trial research.
The inclusion of communities and civil society in the research enterprise is a core principle for ethical conduct of clinical trials, and essential to an effective Global response to HIV. The GPP guidelines have been adapted for clinical trials in other fields, including tuberculosis, COVID-19, and emerging pathogens.
At AVAC, we train trial staff on the guidelines, support their adaptation to other fields, prepare and support advocates to ensure the application of GPP in trial communities, and engage with the research community and ethics boards to maximize their use. Learn more about the GPP Guidelines here.
New! GPP Body of Evidence
Since its first draft, the GPP guidelines have been adopted and used in HIV research and far beyond. AVAC has collected this body of evidence for GPP to demonstrate the power of GPP, to show how GPP can be measured and replicated, and to offer GPP training, tools and connection to everyone involved in the research enterprise.