Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 989
AVAC Infographics for CROI 2026
Going to CROI? AVAC’s up-to-date infographics — on the research & development pipeline, prevention options, and the impact of US funding cuts — are available here for use in conference presentations. We constantly update these graphics, so check back for updates. The latest version of the deck will be available here.
Prevention Option:

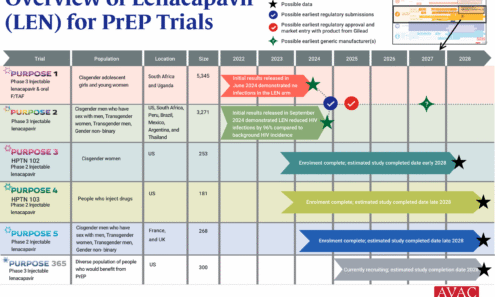
An Overview of Lenacapavir for PrEP Trials
The PURPOSE trials evaluate the safety and efficacy of injectable lenacapavir (LEN), an investigational antiretroviral (ARV) drug being studied as a potential PrEP product. This graphic shows the latest status of all five trials including the groundbreaking results of PURPOSE 1 and PURPOSE 2.
Prevention Option:
AVAC Year in Review 2025
This report highlights AVAC’s role as a trusted voice, a translator of science and catalyst for action and advocacy. It reflects an organization ready for the future: supporting African leadership, strengthening bridges from R&D to delivery and preparing for a new chapter as we move forward into our fourth decade as an organization.
Prevention Option:

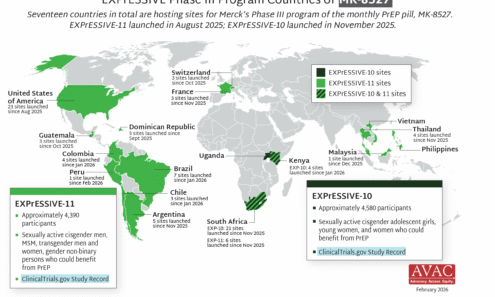
EXPrESSIVE Phase III Program Countries of MK-8527
Seventeen countries are hosting sites for the Phase 3 efficacy trials of a monthly PrEP pill, MK-8527, being developed by Merck (also known as MSD outside of the US and Canada). Merck announced the launch of the Phase 3 trials at IAS 2025 in Kigali. MK-8527 was found to be safe and well-tolerated in Phase 2 clinical trials.
Prevention Option:
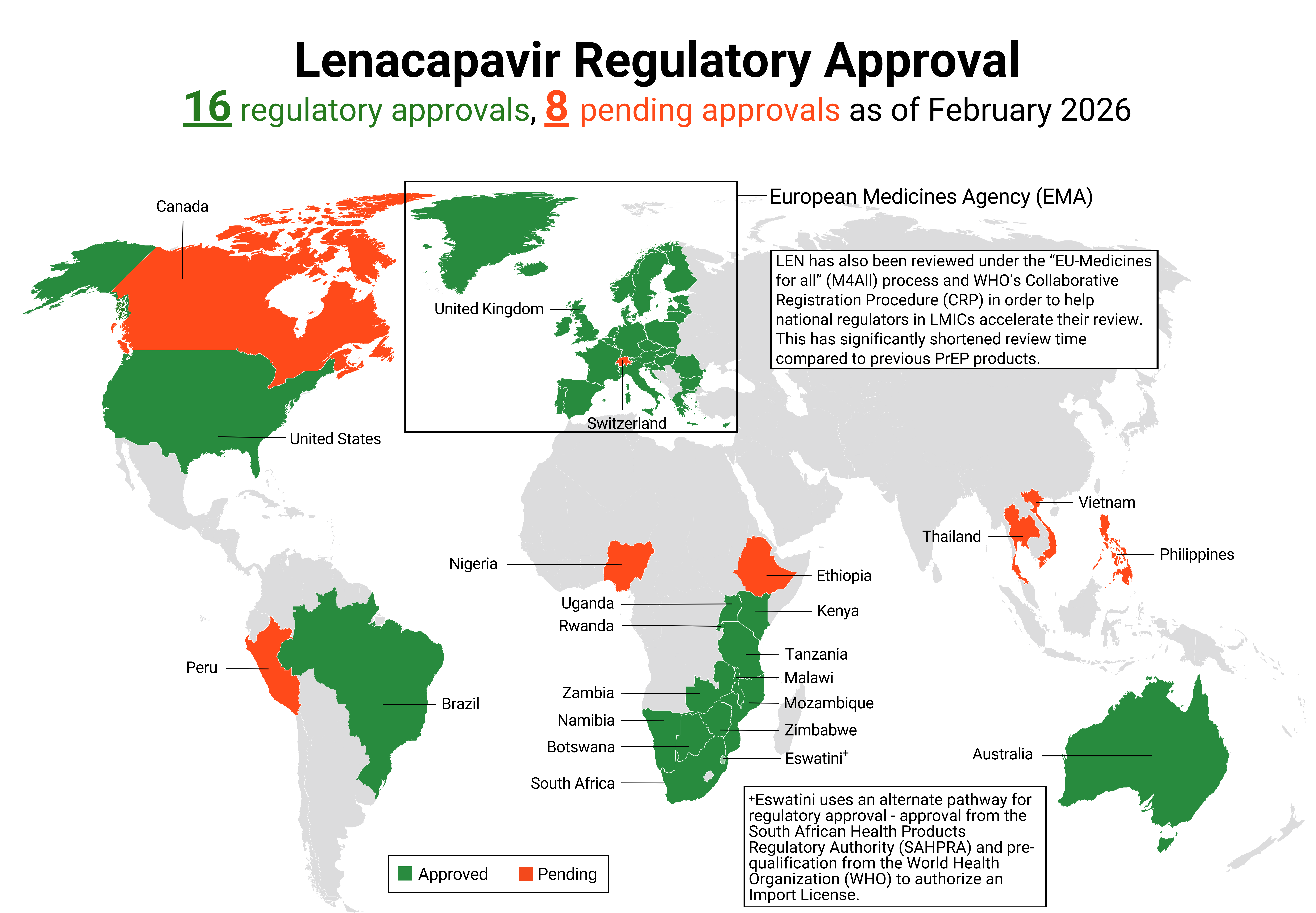
Lenacapavir Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of February 2026.
Prevention Option:
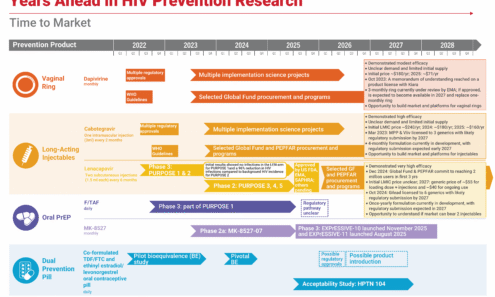
Years Ahead in HIV Prevention Research: Time to Market
This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
Prevention Option:
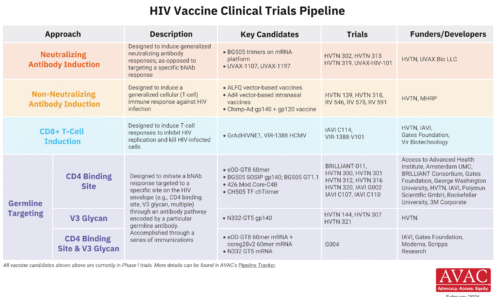
HIV Vaccine Clinical Trials Pipeline
This graphic summarizes the state of HIV vaccine research, detailing the different immunological approaches in clinical trials, the specific candidates being studied, and the collaborative networks of funders and developers working toward an effective vaccine.
Prevention Option:
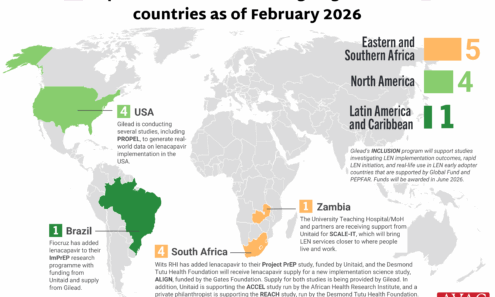
Source of Lenacapavir for PrEP Supply to Early Adopter Countries
The Global Fund, with support from CIFF, and PEPFAR have jointly committed to reaching up to two million people with injectable lenacapavir for PrEP over three years. Supply of LEN began arriving in countries in late 2025 with service delivery planned to start in early 2026.
Prevention Option:

Lenacapavir Implementation Studies
Ongoing and planned implementation studies for the lenacapavir as of February 2026.
Prevention Option:
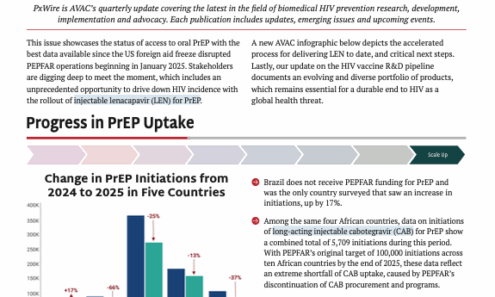
PxWire Volume 16, Issue 1
This issue showcases the status of access to oral PrEP with the best data available since the US foreign aid freeze disrupted PEPFAR operations. Stakeholders are digging deep to meet the moment, which includes an unprecedented opportunity to drive down HIV incidence with the rollout of lenacapavir. Lastly, our update on the HIV vaccine R&D pipeline documents an evolving and diverse portfolio of products, which remains essential for a durable end to HIV as a global health threat.
showing 1-10 of 989