April 24, 2024
Last Week, 1,000+ community advocates, researchers, policy experts, federal public health leadership, medical and service providers from around the US and Puerto Rico attended the NMAC annual Biomedical HIV Prevention Summit in Seattle, Washington. The discussion and debate on PrEP access, especially for racial and ethnic minorities and key populations, PrEP research, care, policy and community-based programs are ones to follow. AVAC’s John Meade (Senior Program Manager, Policy), Jessica Salzwedel (Senior Program Manager: Research Engagement) and Kenyon Farrow (Communications Director) presented in workshops and satellite sessions at the Summit and at the PrEP in Black America preconference

The Summit included sessions that updated community advocates on the latest in biomedical research for new diagnostics, PrEP, PEP, STIs and vaccines. Meade co-presented a Clinical Trials 101 for community advocates to learn more about the research process. Farrow presented an epidemiological overview on HIV and Disparities in the United States for NMAC’s Gay Men of Color Fellows.

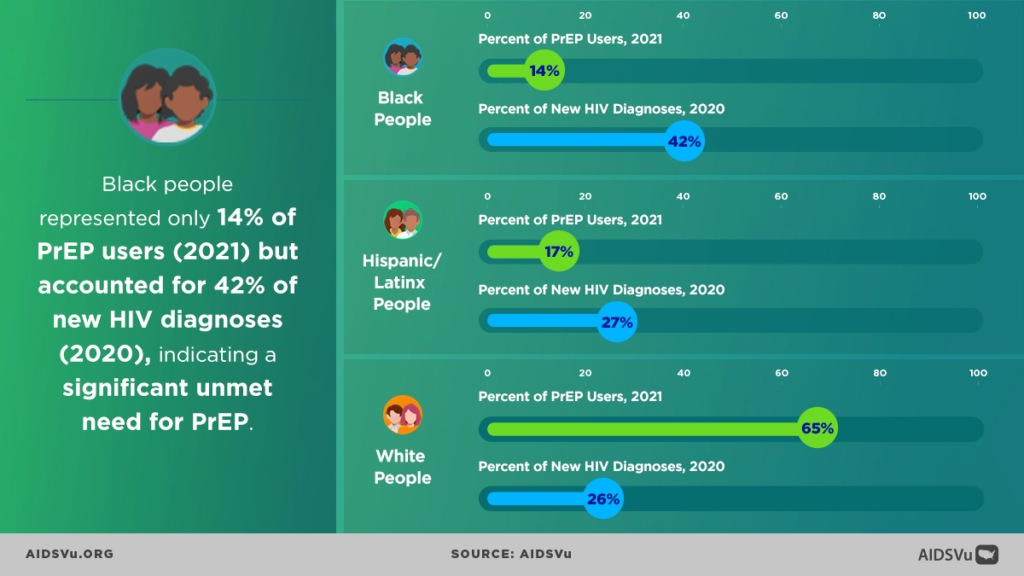
All three AVAC staff contributed to the PrEP in Black America: The State of HIV Prevention Research in the Black Community pre-conference, organized by PrEP in Black America (PIBA). PIBA began in 2022 as a community-led effort to increase Black community mobilization and engagement in PrEP research, policy and access programs. Farrow and Meade are PIBA cofounders, and Meade facilitated the day’s agenda, with more than 200 attendees focused on identifying the research gaps that need to be addressed to increase knowledge, access and use of PrEP. Data shows that while Black people in the U.S. make up 42% of all new HIV diagnoses in 2021, they were only 14% of all PrEP users. By comparison, white Americans are 65% of all PrEP users, but only 26% of all people diagnosed with HIV in the same year.
Salzwedel co-led the closing consensus session at PIBA, a discussion where attendees named research priorities to be later released as part of a National Black-Centered Biomedical HIV Prevention research agenda. One of the most important priorities named, however, goes beyond singling out the right research questions. Attendees showed strong consensus that the research process itself needs to change. Attendees expressed a need and desire for more investment and commitment to community-led research, that can reflect non-traditional ways of gathering data and designing trials and ending the extractive approach that characterizes conventional researcher/community relationships.
To stay up to date with PrEP in Black America, follow them on Instagram and Facebook.

Join AVAC, The Choice Agenda, PrEP4All and HIVMA on Friday, April 26 for a special follow-up webinar, We Can’t End HIV in the United States Without Equitable PrEP Access: Strategies for success. Register here.