
HIV prevention research and development (R&D) must remain a top priority in the HIV response, even as the global context for HIV funding, policies, and programs changes. Since the People’s Research Agenda (PRA) was launched in 2024, significant events have occurred, both advancements and setbacks:
Advancements
- Lenacapavir (LEN) has undergone an expedited regulatory approval process, with FDA approval in June 2025, followed by WHO guidelines in July and approvals by the EMA and multiple African countries between August and December. Plus, agreements with generic manufacturers are now in place to accelerate the introduction of LEN at lower prices beginning in 2027.
- An investigational, once-monthly oral pill for HIV pre-exposure prophylaxis (PrEP) has entered Phase III efficacy testing under the EXPrESSIVE-10 and -11 trials in 17 countries, marking a major step forward in next-generation oral HIV prevention.
- The HIV Vaccine Trials Network (HVTN) launched six small-scale “discovery medicine” trials testing early, experimental vaccine candidates, in the face of ongoing shifts at the US National Institutes of Health (NIH).
- African Clinical Research Network (ACRN), IAVI and additional research partners Ragon and ReiThera initiated C114, a Phase I trial in Zimbabwe and South Africa, testing a gorilla-adenovirus vector–based vaccine containing networked T-cell epitopes designed to elicit strong CD8 T-cell responses.
Setbacks
- Several promising trials and research programs were terminated, most notably USAID-funded HIV projects ADVANCE, BRILLIANT, MATRIX, and MOSAIC, researching early phase vaccines and novel ARV-based and multi-purpose products, as well as real-world implementation of multiple PrEP options. While a scaled down version of the BRILLIANT program will continue with alternate funding, these terminations create a gap in innovative, African-led HIV prevention research, and end any PEPFAR support for HIV R&D after decades of advancement.
- The US NIH has cancelled a range of mRNA vaccine programs and posed threats to the Center for HIV/AIDS Vaccine Development (CHAVD) consortia.
- The funding landscape in which HIV prevention R&D occurs has significantly shifted due to cuts in foreign aid, US government shutdowns, and other political influences.
- Political actions have further destabilized the field; on February 7, 2025, the US Administration issued Executive Order #140204, halting US funding to South Africa and extinguishing prospects for future HIV prevention research support.
The field must face the reality of the new landscape for HIV prevention R&D head-on.
The days of a broad pipeline are behind us. The future depends on sharper priorities, smarter investments, and a portfolio of products that reflect what is needed and realistic to effectively advance HIV prevention and achieve impact against the epidemic. Every dollar (rand, shilling, euro or kwacha), every trial, and every product development go/no-go decision must be weighed for maximum public health impact and equity.
This means building and defending a scientific pipeline that is optimized to deliver impact, including: investments in basic science, discovery medicine, and early-phase trials, while also funding late-stage trials, product development, community engagement and implementation science to drive access to new, evidence-based products. Ultimately, we need a pipeline of viable and effective HIV prevention options for the people who most need them.
The People’s Research Agenda (PRA) was developed throughout 2024 in partnership with global advocates and communities. It proposes a people-centered framework for how HIV prevention products should be developed, tested, and introduced so that products meet the needs, preferences, and realities of the people most affected by HIV.
Now, AVAC is issuing the PRA’s first annual update, the 2025 PRA. To further ensure the PRA is a living document, the new interactive format provides an actionable tracker, an advocacy toolkit, and clear call to action for funders, researchers, advocates, communities, and policymakers to meet this critical moment.
Tracking What Matters
Accountability begins with accurate and accessible information. The PRA focuses on what products are moving forward, which are stalled, and which are at risk of disappearing altogether. By mapping progress across the prevention landscape, the PRA shows where investments align—or fail to align—with community priorities. By tracking the science, the PRA can reveal critical gaps in the ultimate “basket” of prevention products available to meet the needs of diverse populations.
More than a report and data tool, the PRA serves as a sentry for equity, a living accountability mechanism that seeks to transform information into influence and ensure that no community, or promising idea, is left behind.
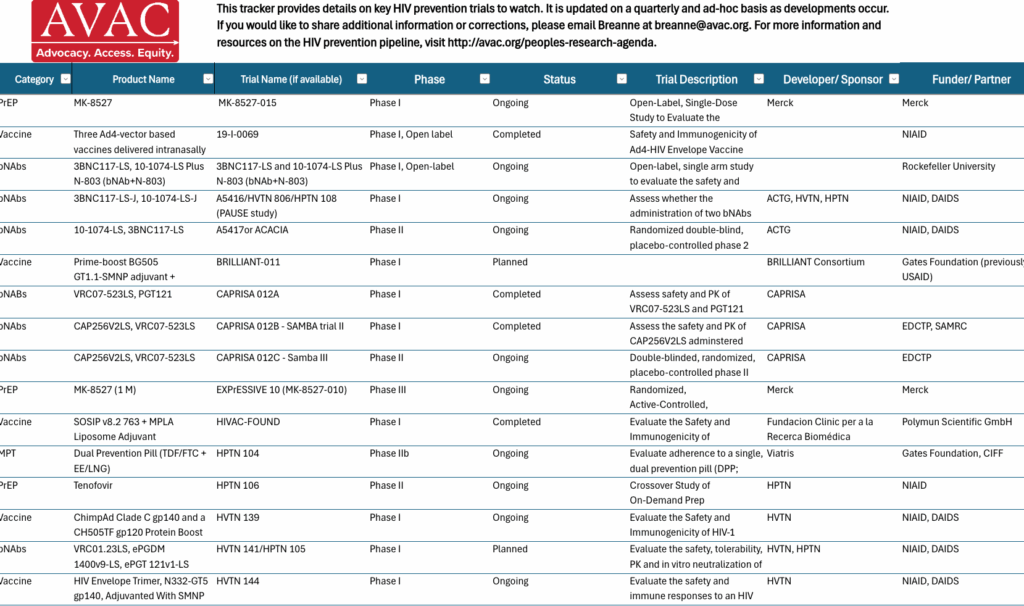
PRA Pipeline Tracker
This tracker provides details on key HIV prevention trials to watch. It is updated on a quarterly and ad-hoc basis as developments occur. If you would like to share additional information or corrections, please email Breanne at [email protected].
People’s Research Agenda 2025 Update Summary Table
December 2025
Downloadable version available.
| Modality | Status (2025) | Gaps | 2026 Priorities |
|---|---|---|---|
| ARV-Based Prevention |
|
|
|
| MPTs |
|
|
|
| Vaccines |
|
|
|
| bNAbs |
|
|
|
| Cross-cutting Issues |
|
|
|
Previous Edition of the People's Research Agenda
