Multipurpose prevention technologies (MPTs) are products designed to address more than one sexual and reproductive health (SRH) need. Male and female condoms—which protect against pregnancy as well as HIV and other sexually transmitted infections (STIs)—are great examples of MPTs that already exist. Many others are in development.
The Role of MPTs in HIV Prevention
For decades, women, especially young women, have asked for discreet products they can control and that address multiple SRH needs, including prevention of HIV and other STIs, as well as pregnancy prevention. Men who have sex with men (MSM) and transgender individuals have also advocated for products that prevent multiple STIs, including HIV.
Effective, affordable, acceptable, and widely accessible MPTs would save lives and money, improving the health of individuals and communities. Some MPTs are being developed as co-formulations (multiple drugs combined into one product) or co-packaged products (two products administered as a pair). MPTs in development include oral pills, vaginal rings, vaginal films, intra-uterine devices (IUDs), implants, inserts, and gels.
Access to MPTs
Effective access to MPTs will depend on ensuring the programs that one day deliver these products reflect the needs and preferences of end users. Integrating HIV services with sexual and reproductive health will be fundamental to this effort.
The MPT Pipeline
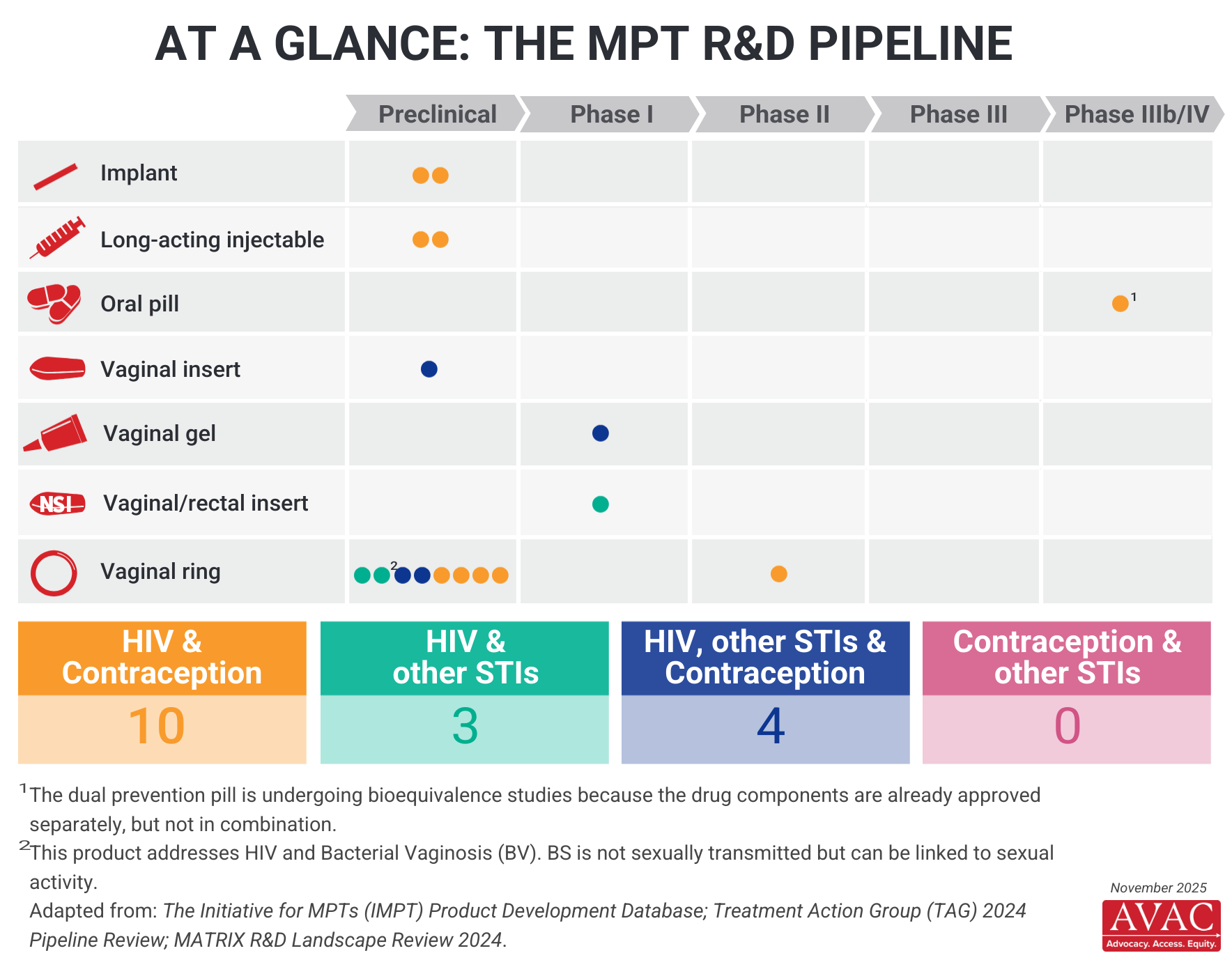
There are many MPT products in pre-clinical and clinical trials but greater diversity in the pipeline is still needed and would represent an important step towards providing all individuals with options to meet their SRH needs as they change across the lifetime. This infographic provides a snapshot of the MPT pipeline.
The MPT furthest along in the research process is the Dual Prevention Pill (DPP)—a daily pill for HIV and pregnancy prevention in cisgender women. The DPP combines TDF/FTC-based oral PrEP with oral contraceptives.
Advocacy for MPTs
Historically, women-centered products have not been an investment or research priority unless pushed by advocates. The field faces a shrinking resource base, and regulatory pathway uncertainty continues to constrain progress. Sustaining scientific investment, advancing regulatory clarity, and gender-responsive design across the R&D is critical to preserve the MPT pipeline’s potential to deliver the next generation of integrated prevention technologies. Coalitions such as CASPR are working to address these needs. Advocacy is crucial to mobilize resources and broad support through all stages of MPT research and development and rollout.
People’s Research Agenda
Our People’s Research Agenda offers a state of the field update on multipurpose technologies. The MPT R&D portfolio remains heavily weighted toward early discover and preclinical research, with few candidates beyond Phase I/II readiness.