Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 976
PxWire Volume 15, Issue 4
This issue provides a range of maps to help orient the field on critical HIV prevention activities: the status of delivering injectable cabotegravir; funders and countries on track for early introduction of injectable lenacapavir; and where new Phase 3 efficacy trials testing MK-8527 are taking place.
Prevention Option:
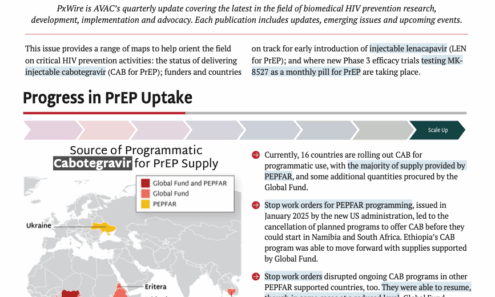
Source of Programmatic Cabotegravir for PrEP Supply
The first supplies of CAB for programmatic use (as opposed to use in implementation studies) began to arrive in countries in 2024. Currently, 16 countries are rolling out CAB for programmatic use, with the majority of supply provided by PEPFAR, and some additional quantities procured by the Global Fund.
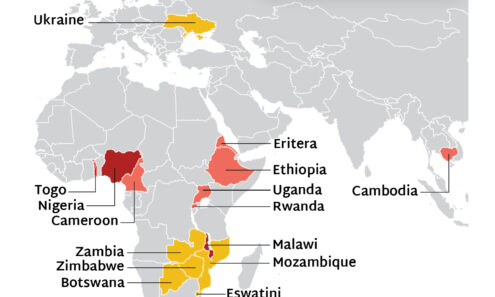
Source of Lenacapavir for PrEP Supply to Early Adopter Countries
The Global Fund, with support from CIFF, and PEPFAR have jointly committed to reaching up to two million people with LEN for PrEP over three years. Supply of LEN is due to begin arriving in countries in late 2025 with service delivery planned to start in early 2026.
Prevention Option:
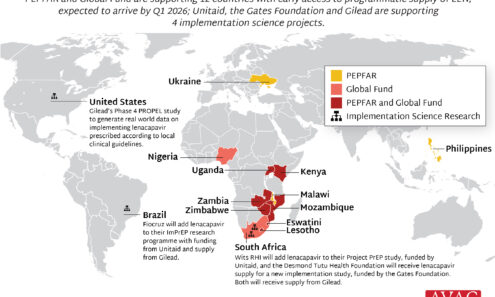
EXPrESSIVE Phase 3 Trials Countries of MK-8527
Seventeen countries are hosting sites for the Phase 3 efficacy trials of a monthly PrEP pill, MK-8527, being developed by Merck (also known as MSD outside of the US and Canada). Merck announced the launch of the Phase 3 trials at IAS 2025 in Kigali. MK-8527 was found to be safe and well-tolerated in Phase 2 clinical trials.
Prevention Option:
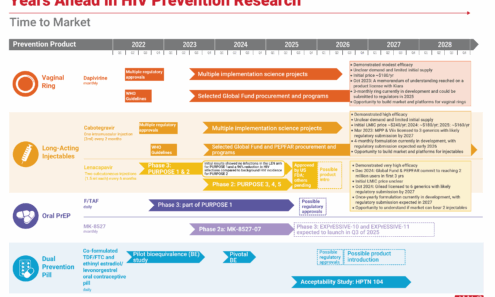
Years Ahead in HIV Prevention Research: Time to Market
This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
Prevention Option:
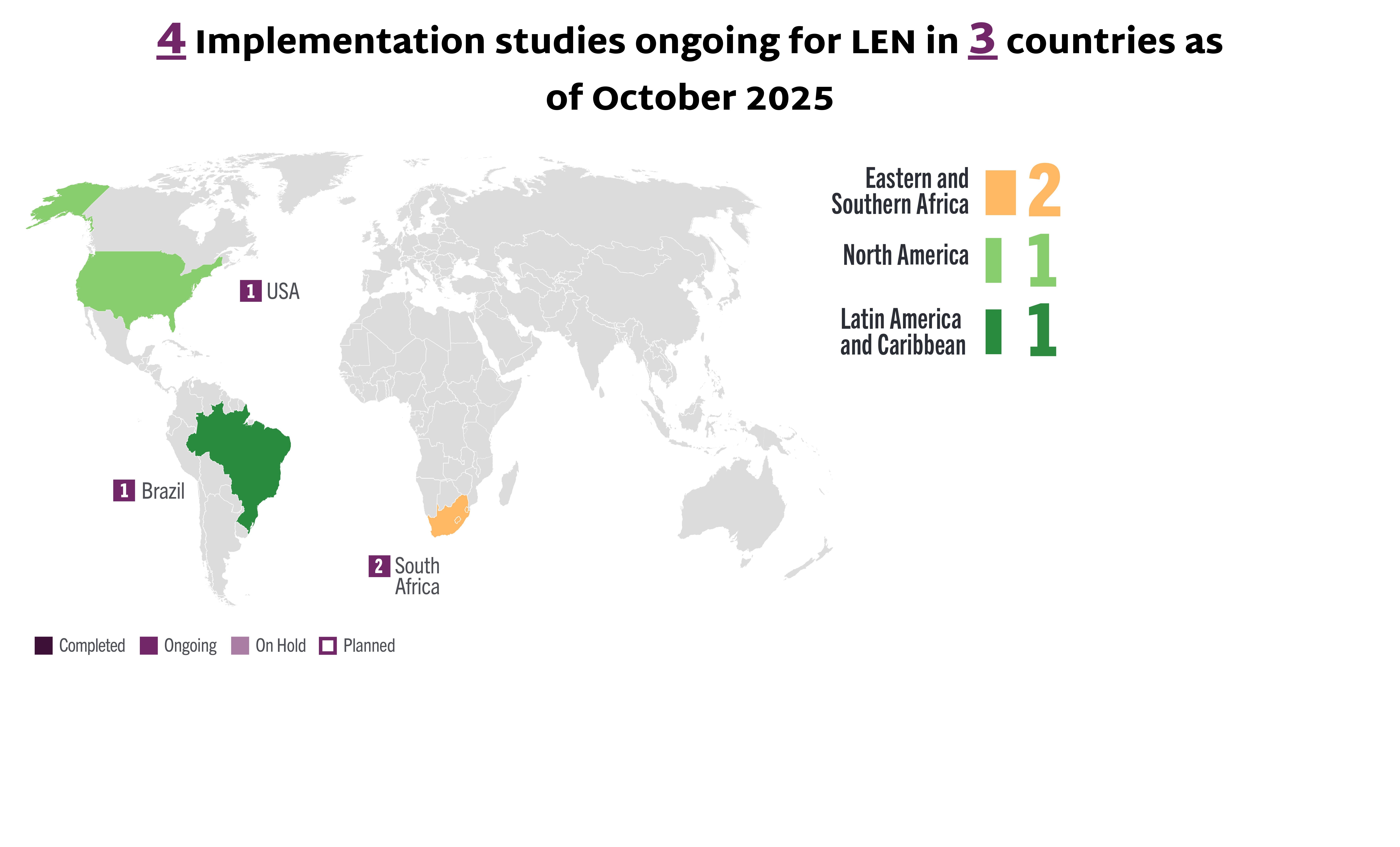
Lenacapavir Implementation Studies
Ongoing and planned implementation studies for the lenacapavir as of October 2025.
Prevention Option:
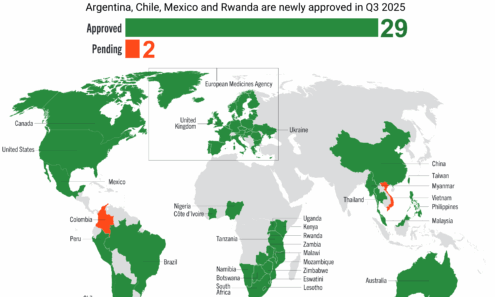
Dapivirine Vaginal Ring Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of October 2025.
Prevention Option:
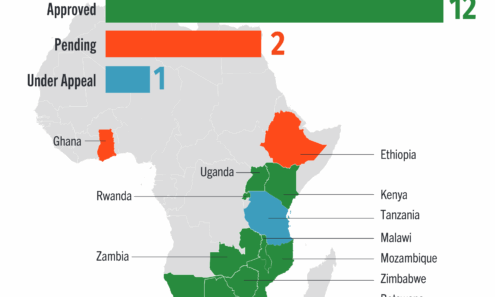
Cabotegravir Regulatory Approval
Regulatory approvals and those pending for cabotegravir as of October 2025.
Prevention Option:
Dapivirine Vaginal Ring Volume
DVR supply available to low- and middle-income countries as of October 2025.
Prevention Option:
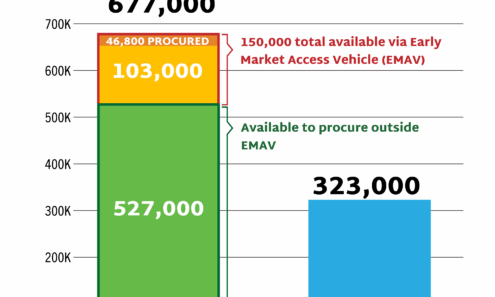
Cabotegravir Volume
Allocation of non-commercial cabotegravir for PrEP supply in low- and middle-income countries, 2023-2026, as of October 2025.
Prevention Option:
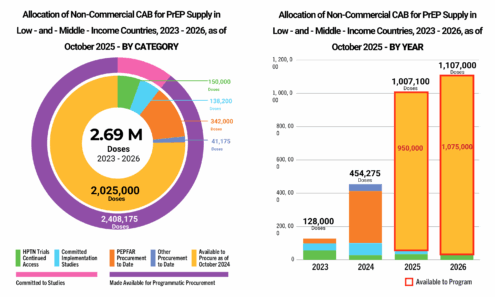
showing 1-10 of 976