Px Wire February 2023
Volume 13, Number 1
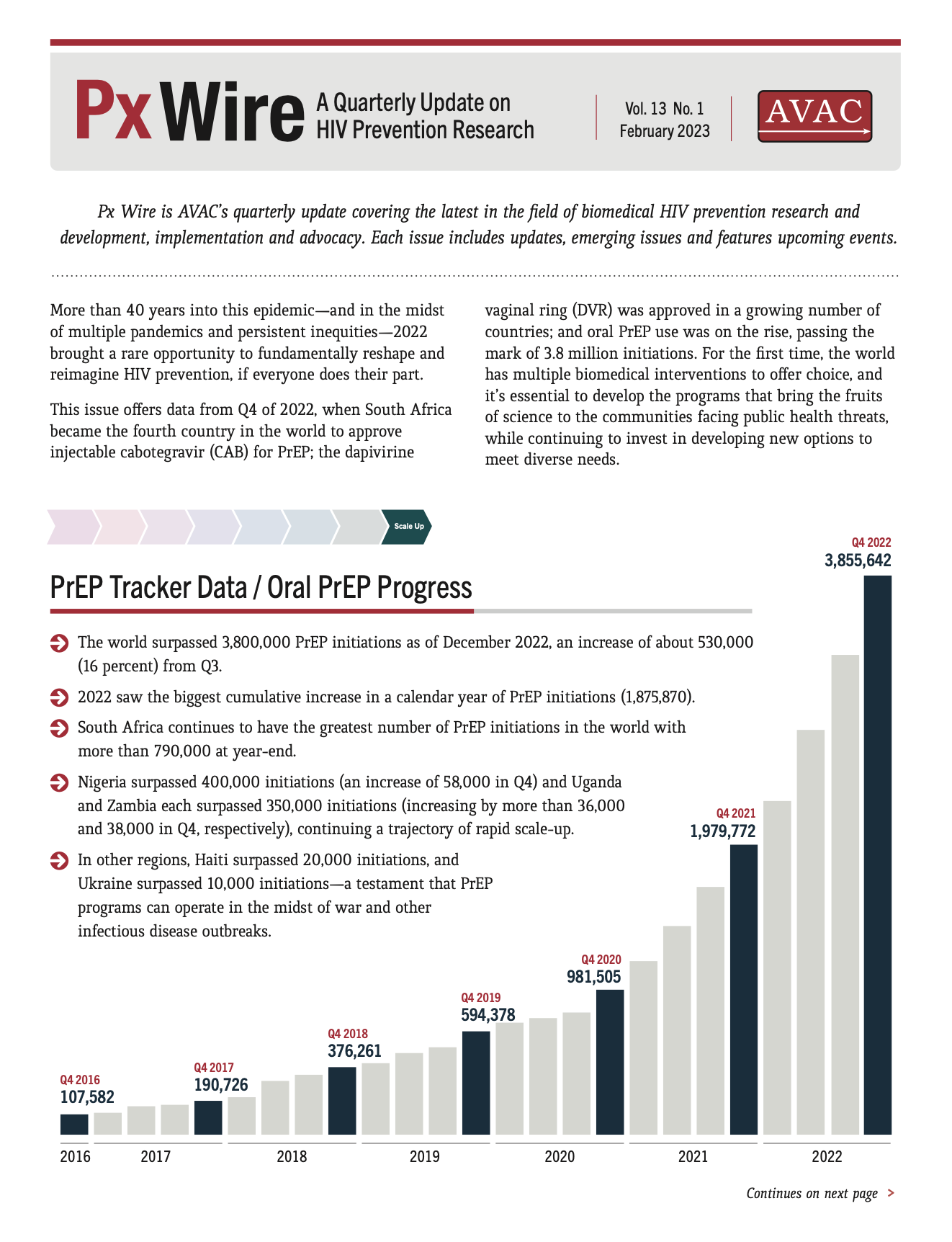
This issue offers data from Q4 of 2022, when South Africa became the fourth country in the world to approve injectable cabotegravir (CAB) for PrEP; the dapivirine vaginal ring (DVR) was approved in a growing number of countries; and oral PrEP use was on the rise, passing the mark of 3.8 million initiations. For the first time, the world has multiple biomedical interventions to offer choice, and it’s essential to develop the programs that bring the fruits of science to the communities facing public health threats, while continuing to invest in developing new options to meet diverse needs.
- Topics:
- Accelerating Product Innovation
Was this content helpful?
Tell us how we can improve the content.
Was this content helpful?
Thank you for your feedback!