Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 972
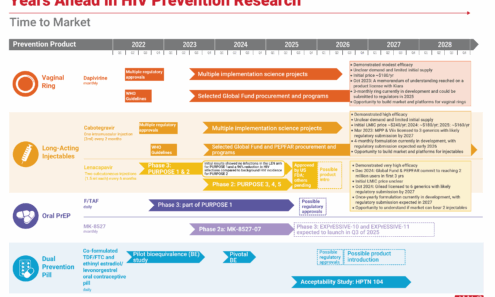
Years Ahead in HIV Prevention Research: Time to Market
This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
Prevention Option:
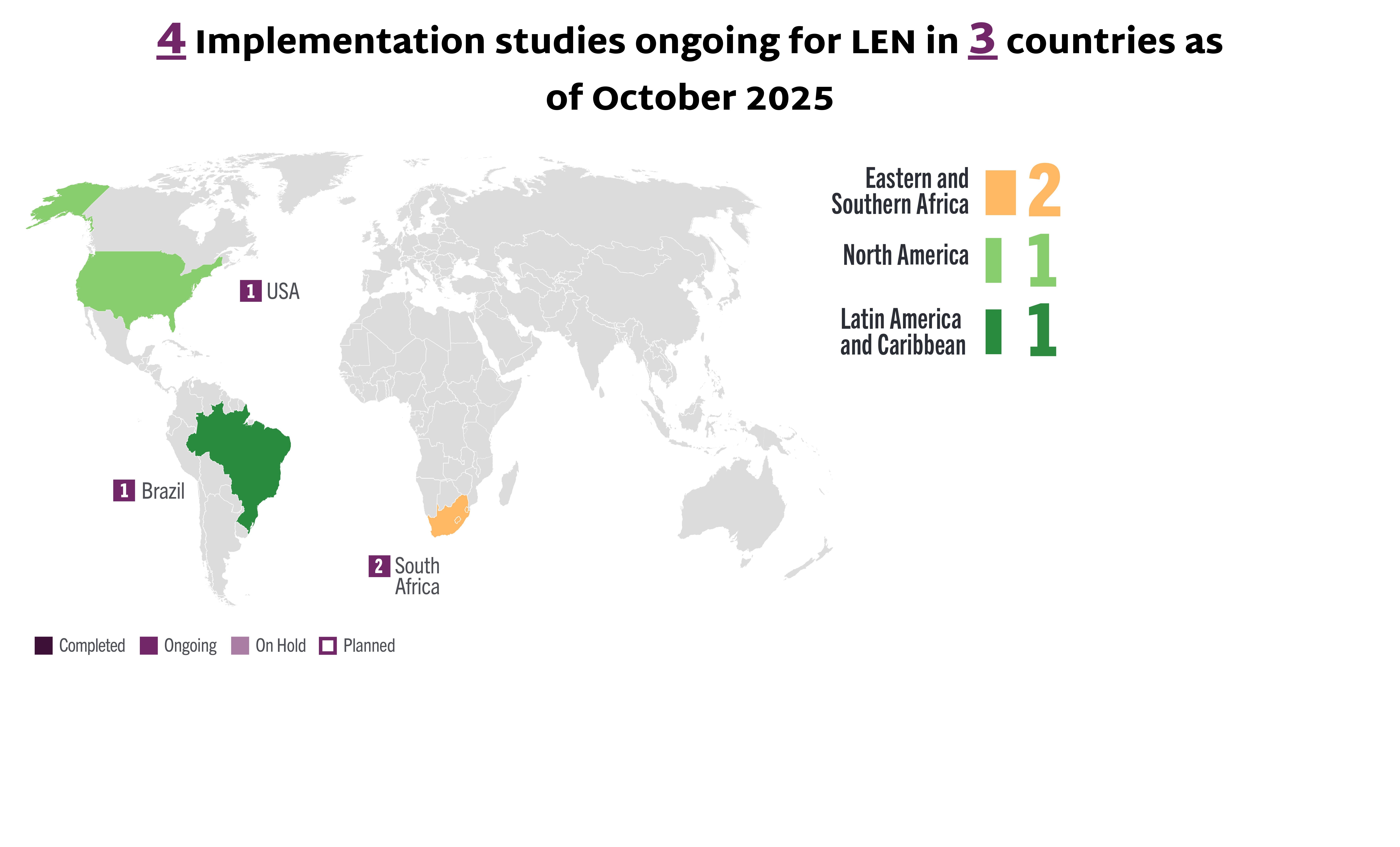
Lenacapavir Implementation Studies
Ongoing and planned implementation studies for the lenacapavir as of October 2025.
Prevention Option:
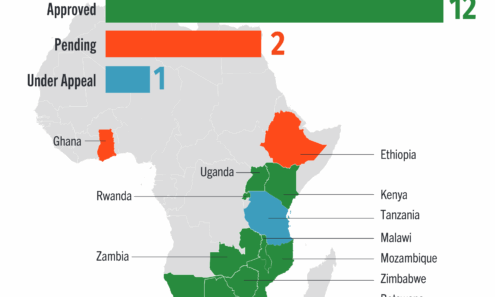
Dapivirine Vaginal Ring Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of October 2025.
Prevention Option:
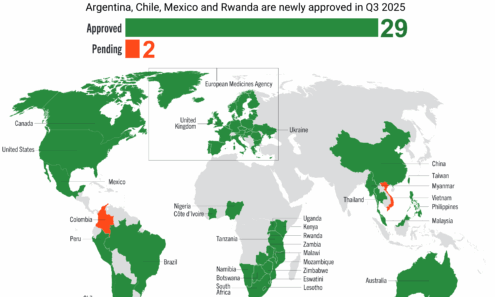
Cabotegravir Regulatory Approval
Regulatory approvals and those pending for cabotegravir as of October 2025.
Prevention Option:
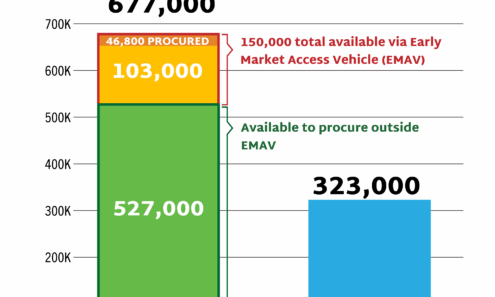
Dapivirine Vaginal Ring Volume
DVR supply available to low- and middle-income countries as of October 2025.
Prevention Option:
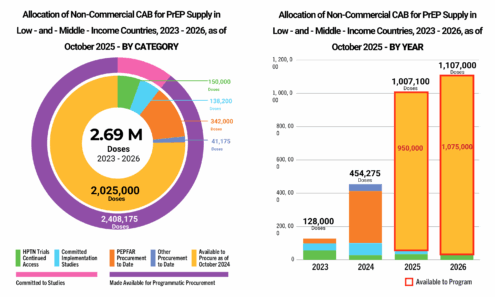
Cabotegravir Volume
Allocation of non-commercial cabotegravir for PrEP supply in low- and middle-income countries, 2023-2026, as of October 2025.
Prevention Option:
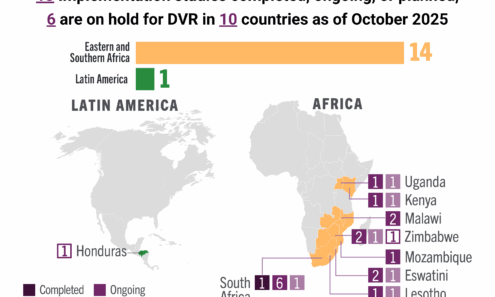
Dapivirine Vaginal Ring Implementation
Ongoing and planned implementation studies for the dapivirine vaginal ring as of October 2025.
Prevention Option:
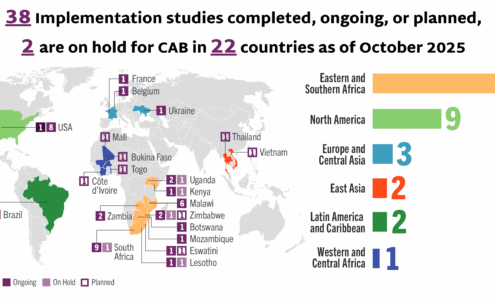
Cabotegravir Implementation
Implementation studies completed, ongoing, or planned for cabotegravir as of October 2025.
Prevention Option:
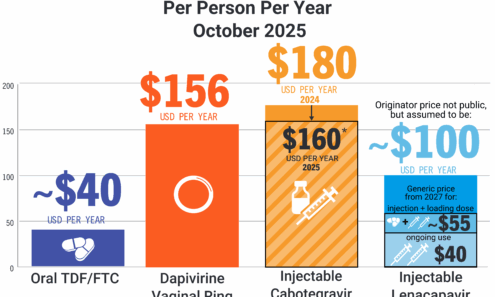
PrEP Price Comparison
Comparing the annual price of oral TDF/FTC vs. the dapivirine vaginal ring and injectable cabotegravir.
Prevention Option:
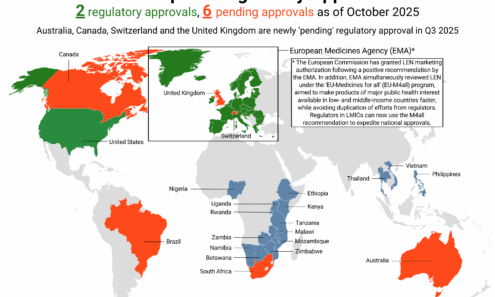
Lenacapavir Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of October 2025.
Prevention Option:
showing 1-10 of 972