Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 987
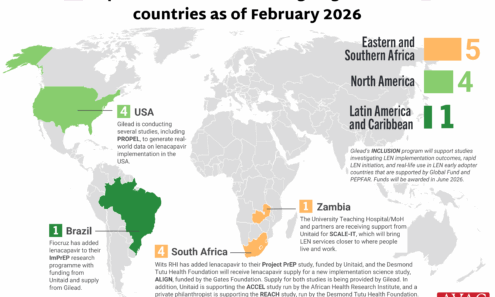
Lenacapavir Implementation Studies
Ongoing and planned implementation studies for the lenacapavir as of February 2026.
Prevention Option:
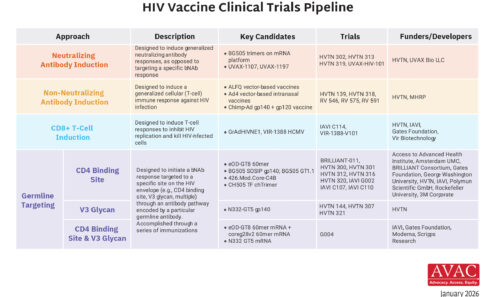
HIV Vaccine Clinical Trials Pipeline
This graphic summarizes the state of HIV vaccine research, detailing the different immunological approaches in clinical trials, the specific candidates being studied, and the collaborative networks of funders and developers working toward an effective vaccine.
Prevention Option:
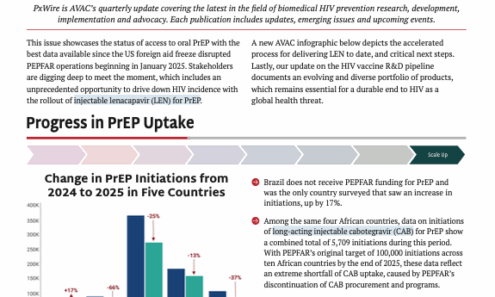
PxWire Volume 16, Issue 1
This issue showcases the status of access to oral PrEP with the best data available since the US foreign aid freeze disrupted PEPFAR operations. Stakeholders are digging deep to meet the moment, which includes an unprecedented opportunity to drive down HIV incidence with the rollout of lenacapavir. Lastly, our update on the HIV vaccine R&D pipeline documents an evolving and diverse portfolio of products, which remains essential for a durable end to HIV as a global health threat.
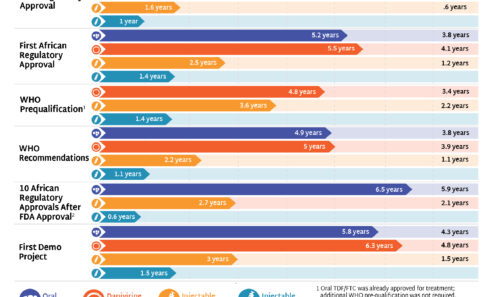
Speeding Up Access to PrEP
The timeline for hitting key milestones in product introduction is moving faster for injectable LEN than for any previous PrEP products, starting from the announcement of efficacy results in Phase III trials. LEN’s accelerated timeline compared to oral PrEP, DVR, and CAB reflects a field-wide effort to learn lessons from previous PrEP rollout and not repeat the mistakes of the past.
Prevention Option:
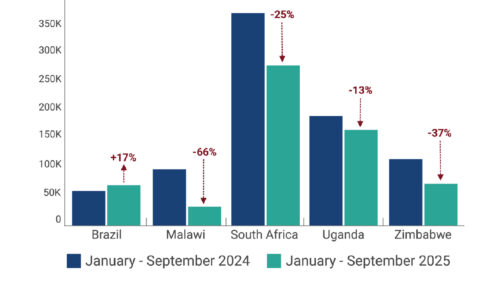
Change in PrEP Initiations from 2024 to 2025 in Five Countries
Between the period January-September 2024 to January-September 2025, PrEP initiations fell between 13% and 66% in selected high-volume PrEP countries where ministries of health (MoH) were able to provide data. Among the five countries depicted, four saw significant decline in PrEP uptake. All four relied on PEPFAR PrEP programs and were disrupted by stop work orders (SWO).
Prevention Option:
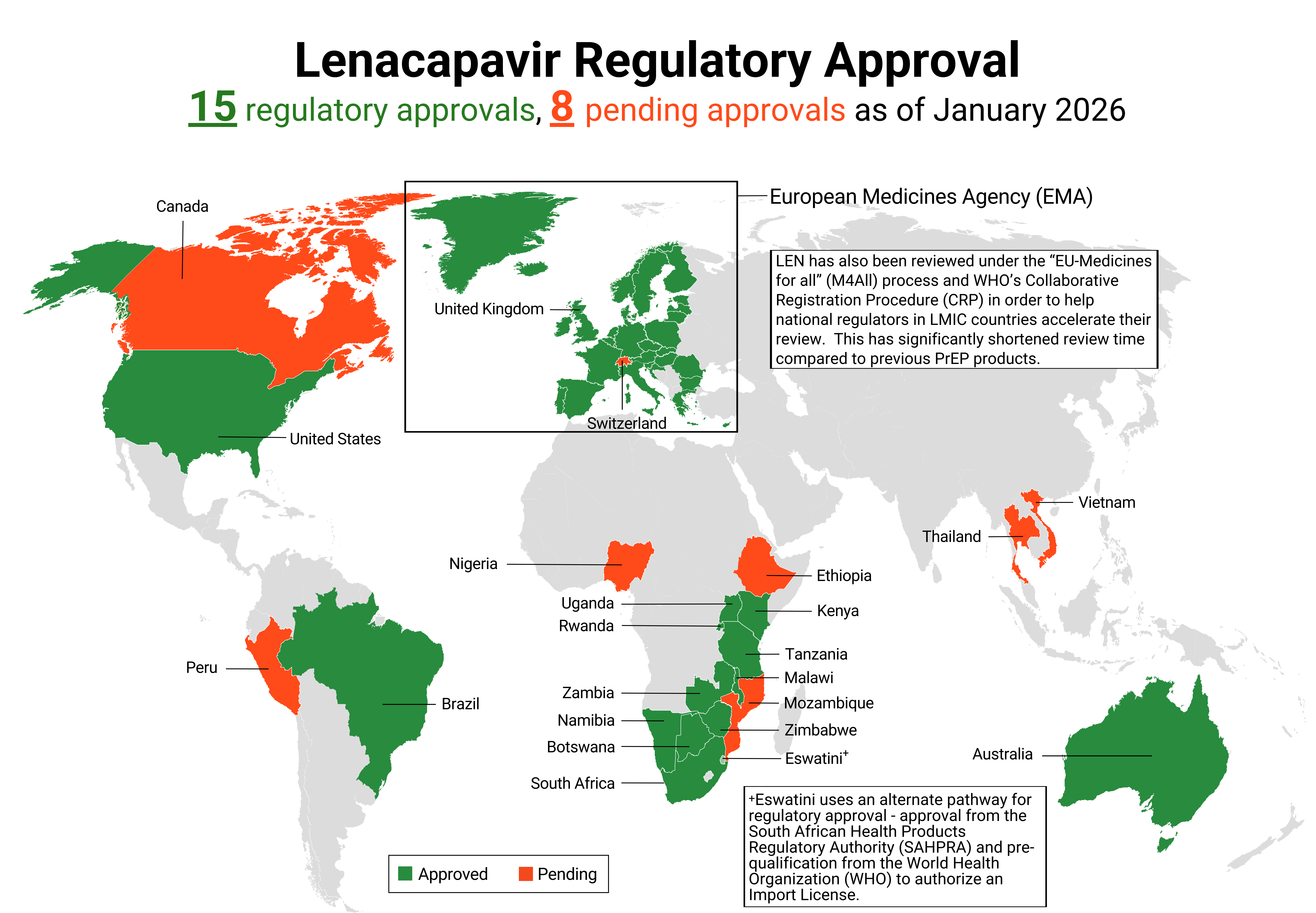
Lenacapavir Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of January 2026.
Prevention Option:
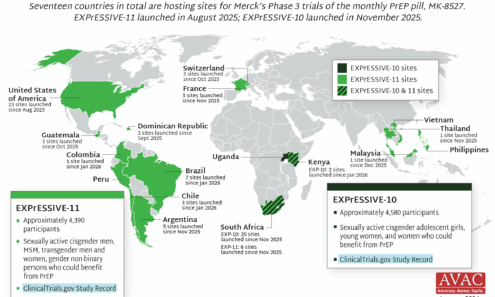
EXPrESSIVE Phase 3 Trial Countries of MK-8527
Seventeen countries are hosting sites for the Phase 3 efficacy trials of a monthly PrEP pill, MK-8527, being developed by Merck (also known as MSD outside of the US and Canada). Merck announced the launch of the Phase 3 trials at IAS 2025 in Kigali. MK-8527 was found to be safe and well-tolerated in Phase 2 clinical trials.
Prevention Option:
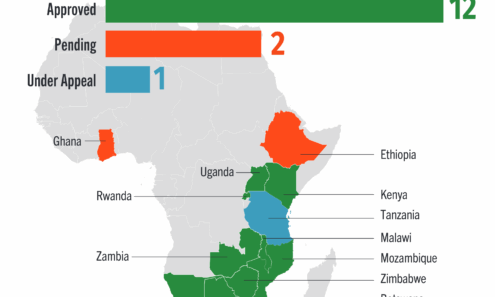
Dapivirine Vaginal Ring Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of January 2026.
Prevention Option:
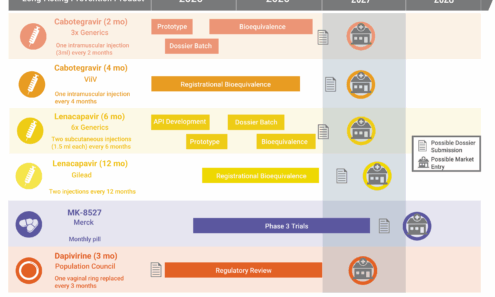
An “Innovation Pile-Up” in Next-Generation LA-PrEP is Possible
The HIV prevention market is headed toward a period of significant opportunity—and possible congestion—as a slate of new products are on track for continued development and potential introduction to the market in 2027 and 2028. Markets and policies must be built to support the products in the market already, so that new options can be rapidly deployed and deliver impact. Otherwise, the field will squander time and money, with epidemic control slipping further out of reach.
Prevention Option:
Source of Lenacapavir for PrEP Supply to Early Adopter Countries
The Global Fund, with support from CIFF, and PEPFAR have jointly committed to reaching up to two million people with injectable lenacapavir for PrEP over three years. Supply of LEN began arriving in countries in late 2025 with service delivery planned to start in early 2026.
Prevention Option:

showing 1-10 of 987