Antiretroviral drugs (ARVs) were originally developed to treat people living with HIV. But research has proven that ARVs can also prevent HIV acquisition among individuals who are HIV-negative. This is called pre-exposure prophylaxis, or PrEP, and it’s a vital tool in HIV prevention.
PrEP Delivery
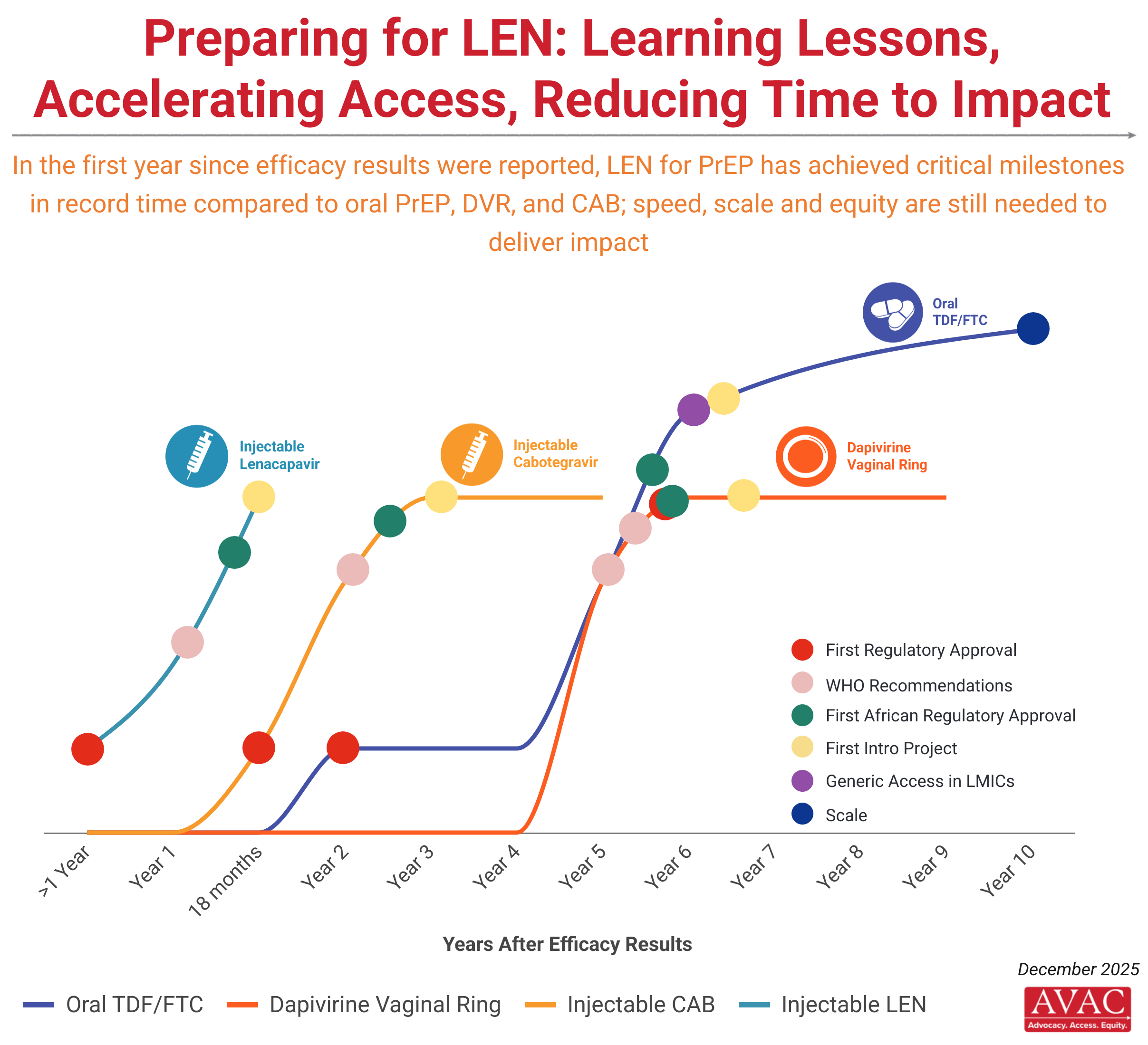
The first daily PrEP pill was approved by the FDA in 2012, but rollout was slow, uncoordinated and poorly planned. Uptake is nowhere near where it should be to address the HIV epidemic. While PrEP won’t be right for everyone at risk, the next generation of products—some entering the market now—must act on the lessons from oral PrEP rollout, by improving coordination and speeding access.
The rollout of injectable lenacapavir (LEN for PrEP), injectable cabotegravir (CAB for PrEP) and the dapivirine vaginal ring (DVR), the latest prevention tools to be proven effective, represents an opportunity to get rollout right.
Approved Options
- Lenacapavir (a six monthly injectable) showed 100% efficacy in the PURPOSE 1 trial and 96% efficacy in the PURPOSE 2 trial, compared to daily oral PrEP. LEN for PrEP was approved by the FDA in June 2025. To learn all about lenacapavir and for related AVAC resources, click here.
- Cabotegravir (a two monthly injectable) became the first injectable PrEP option when it gained regulatory approval in 2021. Learn more about injectable CAB for PrEP on PrEPWatch.org.
- The dapivirine vaginal ring (a monthly vaginal ring) received a positive opinion from the European Medicines Agency in 2020. Learn more about the ring on PrEPWatch.org.
- F/TAF (brand name Descovy) became the second daily oral PrEP pill to be approved in 2019, but only for men who have sex with men (MSM) and transgender women. There is not yet sufficient evidence of efficacy among cisgender women. Learn more about the PURPOSE 1 trial that tested F/TAF among women.
- The daily pill TDF/FTC (brand name Truvada) was first approved for use as oral PrEP in 2012. Now dozens of countries have approved it and launched programs to deliver it. But PrEP still has not reached many of the people who need it most. Find where to access PrEP in your country.
PrEP in Development: What’s in the pipeline?
The pipeline of ARV-based prevention has significant momentum in long-acting innovations, with scientific advances across both injectable and oral delivery platforms.
Further along in development are a monthly oral pill (MK-8527), a 3-monthly ring, a multi-purpose technology that combines PrEP with a contraceptive that prevents pregnancy (the Dual Prevention Pill), and a four-monthly and annual injectables. To learn more about these products go to prepwatch.org/products. Meanwhile, Early-stage studies of multipurpose prevention technologies (MPTs), implants, and on-demand regimens are stalling due to funding cuts, threatening the future diversity of the HIV prevention pipeline.
PrEP Advocacy
Sustained investment, strategic prioritization, and early access planning are essential to ensure that basic science and innovation in early phase R&D ultimately expands, not narrows, real prevention choice for women, adolescent girls, and all people affected by HIV.
The emergence of multiple options simultaneously raises opportunities, while presenting critical policy and programmatic questions about how governments will prioritize and allocate resources while safeguarding user preferences and choice. AVAC supports a range of programs to advance this advocacy. We track the status of rollout and uptake of PrEP and R&D of upstream research. We call for continued investment in R&D for new options; support engagement between researchers, donors, community leaders, government, and civil society in the design of trials investigating new PrEP options and the design of programs to deliver PrEP effectively to everyone who needs it; and participate in collaborations to hold decision-makers accountable to commitments on funding, policy, and implementation.
Find out more about PrEP access and rollout across the globe at AVAC’s PrEPWatch.
People’s Research Agenda
Our People’s Research Agenda offers a state of the field update on ARV-based prevention, which has seen significant momentum in long-acting innovations, with scientific advances across both injectable and oral delivery platforms.